Greenhouse gases work by absorbing heat emitted from Earth’s surface. This is known as the Greenhouse Effect and it keeps Earth warmer than it otherwise would be.
Greenhouse gases are naturally present in Earth’s atmosphere, and I’ll assume that you already know a bit about them. I have a post on this here, if you’d like more of an introduction.
Greenhouse gases change Earth’s climate through the Greenhouse Effect. So, to understand how greenhouse gases work, you first need to understand that.
What is the Greenhouse Effect?
The natural Greenhouse Effect is a good thing. It keeps Earth warm enough for things to live on it. Earth’s average surface temperature is 15 °C. Without the greenhouse effect, it would be about –18 °C.
The Greenhouse Effect is the result of a few different processes. If I had to summarise it in a couple of sentences, I’d say this: Earth absorbs energy from the Sun, heats up and releases its own energy back out to space. Greenhouse gases hold some of this energy back, which keeps Earth warm.
If you want to know how it works in more detail, one of the best ways is with a diagram. Luckily, I have drawn you one below.
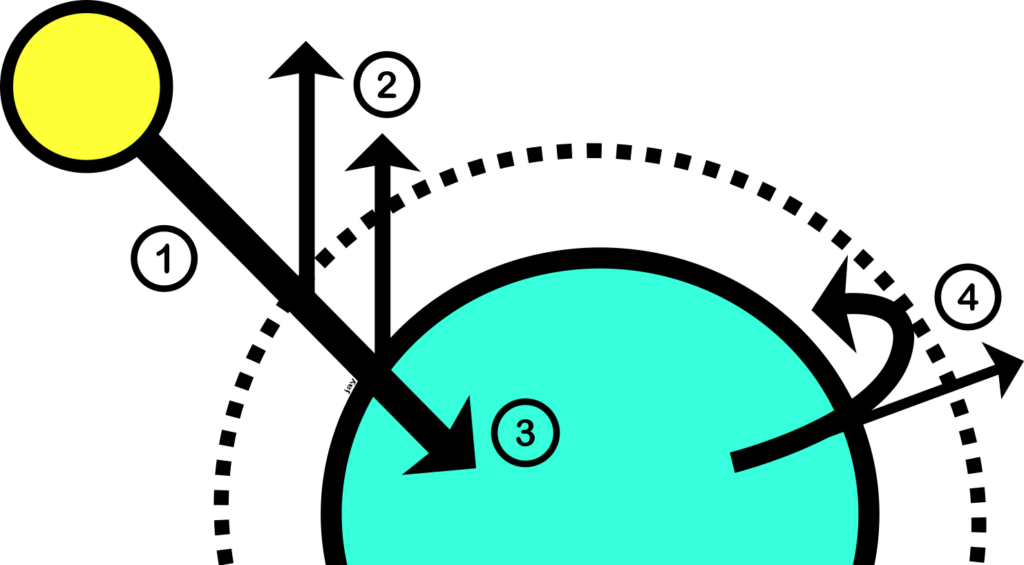
Part 1 of my diagram starts with the Sun on the left. It looks small because it’s far away. Energy from the Sun travels to Earth, which I’ll assume you’re already happy with because of things like daylight and sunburn.
In part 2, you can see that some of the Sun’s energy is reflected away from Earth. This happens when, sunlight bounces off surfaces such as clouds or snow. About 30% of the incoming energy is reflected.
Part 3 shows the part of the Sun’s energy that is absorbed by surfaces on Earth, such as land and sea. This causes them to heat up.
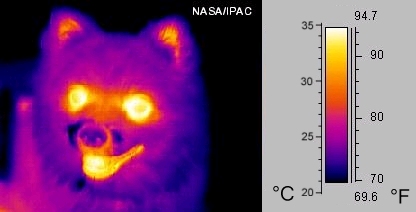
Part 4 shows Earth’s surfaces releasing energy back into space. This happens because warm surfaces emit their own energy. You are a warm object, so you’re also emitting your own energy. This energy is mainly infrared radiation. You might have seen images from infrared cameras, which can detect the infrared radiation emitted by warm objects. See, for example, the image of the warm dog below. Earth’s warm surfaces also emit infrared radiation, and this travels back into space.
Some of the radiation emitted by Earth’s warm surfaces doesn’t make it back into space, and this is where greenhouse gases come in. When these surfaces emit energy, greenhouse gases absorb some of it and stop it from reaching space. The energy is re-emitted in all directions, including back towards Earth’s surface, keeping it warm. This effect is similar to the way an actual greenhouse works, hence the name.
Can we see the greenhouse effect happening?
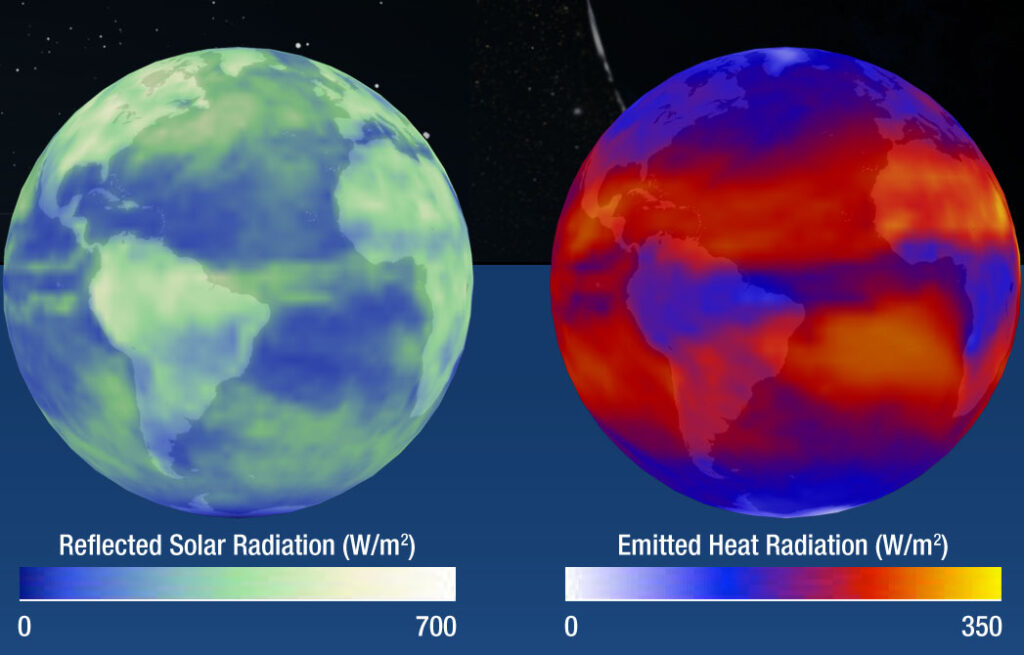
Yes and no. Greenhouse gases are colourless, and many of the processes in the Greenhouse Effect happen very high up. Handily, we can see some of them from space using similar technology to the camera that took the photo above of the dog. See, for example, the images below from NASA. They show the solar energy reflected by Earth (left, and part 2 of my diagram), and the energy emitted by Earth back into space (right, and part 4 of my diagram).
Note that the NASA images refer to the reflected and emitted energy as radiation, because that’s the more scientific name for it. The coloured bars at the bottom show which parts of Earth reflect or emit the most energy, and the units (W/m2) measure the intensity of the energy emitted. However, you don’t need to know that to understand the image. It’s enough to know that a higher number means more energy reflected or emitted.
Are all gases greenhouse gases?
No. Scientifically speaking, all greenhouse gases are molecules made of atoms joined by chemical bonds. For example, carbon dioxide is made of one carbon atom chemically bonded to two oxygen atoms. It’s these chemical bonds that cause the action of greenhouse gases.
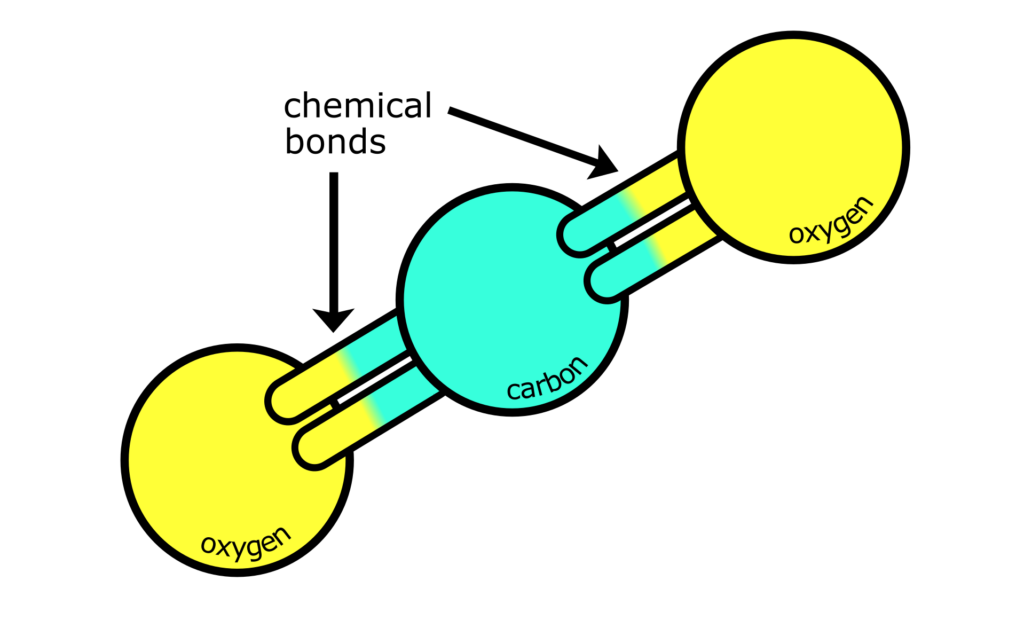
Only some types of chemical bonds absorb the energy emitted by Earth’s surfaces. Essentially, the chemical bonds in greenhouse gases are a good match for the energy emitted by Earth, whereas the bonds in other gases are not. If that’s enough of an explanation for you then great, we’re pretty much done here. If not, keep reading, we’re about to get technical.
Why are some gases greenhouse gases whilst others are not?
To understand this properly you need to know about frequencies of emitted radiation and bond vibration. That sounds more serious than it is. You can think of the energy absorbed and emitted by Earth as a type of wave. (Because it is. It’s an electromagnetic wave.) Like all waves, the emitted radiation has a frequency, which is the number of waves that travel past a point each second.
Your voice, which also emits energy in waves, has a frequency of a hundred or so waves per second. The energy emitted by Earth has a frequency of about 0.3 – 430 trillion waves per second (THz). That’s between 300,000,000,000 and 430,000,000,000,000 waves each second. It’s faster than a hyped-up hamster on wheel.
Let’s go back to the chemical bonds in gases. All chemical bonds naturally move a bit. This is called bond vibration, and it also has a frequency. If a chemical bond meets an electromagnetic wave with the same frequency as its natural vibration, it will absorb the wave.
The frequency of the bond vibration depends on the length and strength of the bond, and the type of atoms in the bond. It happens to be the case that the frequencies absorbed by the bonds in greenhouse gases nicely match those emitted by Earth, which, remember, are in the range 0.3 – 430 THz.
| Greenhouse gas | Frequency absorbed (THz) |
| carbon dioxide | 70.4 |
| methane | 85.4 – 88.4 |
| water | 89.9 – 113.9 |
| dichlorodifluoromethane | 30.0 – 42.0 |
I’d give you a reference for the absorption ranges of these gases, but I got them from one of my A-level chemistry textbooks so I’m afraid you’re out of luck.
Regardless, you should be able to see that the frequencies absorbed by the gases nicely fit into the range of radiation emitted by Earth. This means that greenhouse gases can catch the energy emitted by Earth’s warmed surface and prevent it from quickly pissing off into space.
Other gases in Earth’s atmosphere (mainly oxygen and nitrogen) do not absorb infrared frequencies. This means they cannot catch Earth’s emitted energy and consequently are not greenhouse gases.