The hydrogen industry is growing, with encouragement from governments, in the pursuit of decarbonization. The transport sector has flirted with hydrogen for decades. However, hydrogen is a problematic solution for low-carbon transport. It has the lowest energy density per unit volume of any fuel, and a high energy cost to manufacture and store. It will need to compete with other solutions which may offer greater efficiency and lower economic cost.
Conversations about hydrogen as an alternative fuel have been around for decades. I used to have heated family discussions about whether the future of the car industry would be hydrogen or electric. My cousin, firmly in the hydrogen camp, claimed there was no future for electric. He now works for Tesla.
Let’s look at the current state of play. In recent years, policy makers in many countries including the UK have released strategy documents showing their intentions for the coming decades. The UK government is seeking to make the country a leader in developing hydrogen as a domestic fuel source, calling it a “super-fuel”. Plans for a 20% blend of hydrogen into the national gas grid are under consultation, and models for developing a hydrogen economy through hydrogen transport and storage infrastructure are to be in place by 2025. The goal is an output of 10 GW of hydrogen fuel by 2030, of which at least half should be from electrolysis (I’ll get to that in a bit).
Here we’ll consider the use of hydrogen in the transport sector. Its use in other sectors, such as energy storage or home heating, or its extension to a wider ‘hydrogen economy’, deserve to be considered separately.
Hydrogen as a transport fuel for cars

The UK hydrogen strategy tells us that hydrogen is likely to be fundamental to achieving net zero in transport, where it may provide alternatives to petrol and diesel, as well as providing solutions for aviation and shipping.
With that in mind, let’s start with hydrogen-powered cars. Many car manufacturers have already decisively picked electric vehicles over hydrogen technologies for their future strategies, and when you peel back some layers of detail, it’s easy to understand why.
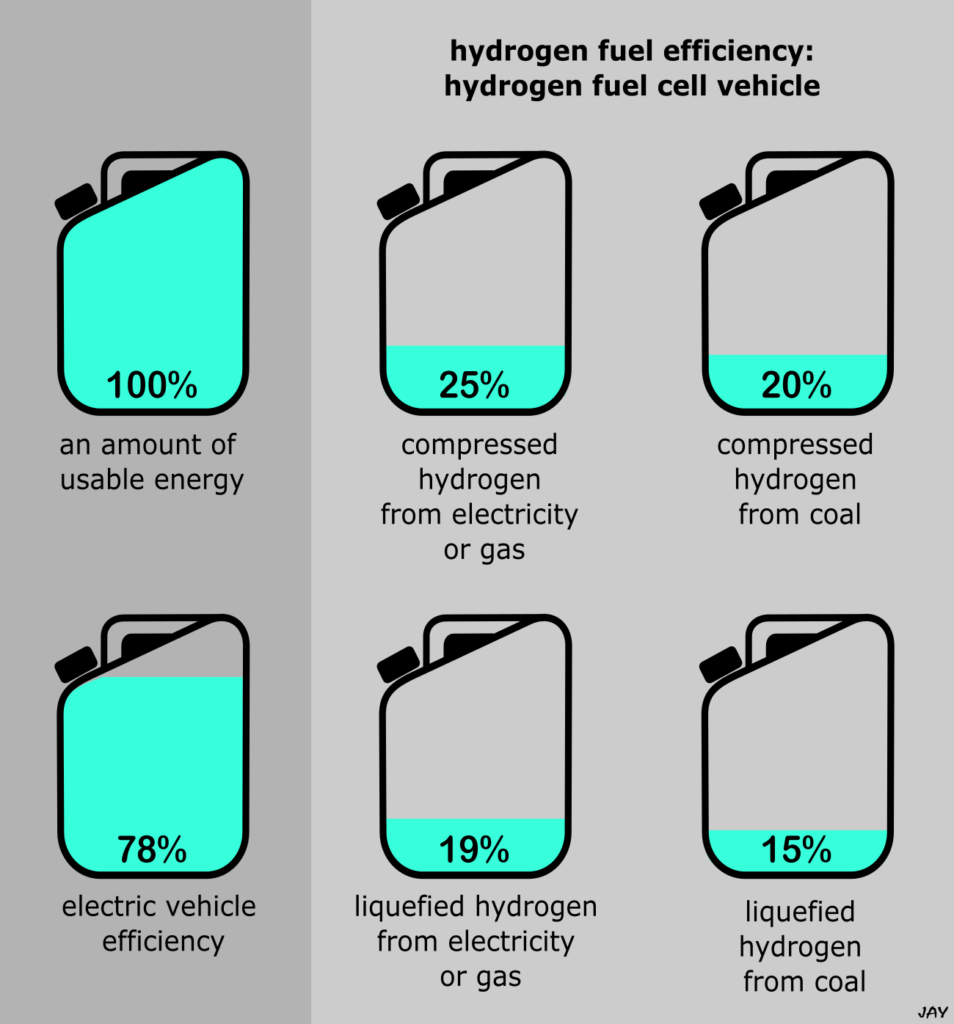
You refuel hydrogen-powered cars in much the same way as petrol/diesel ones. That is, you fill hydrogen into a tank and drive away whilst the car uses the hydrogen to release energy that powers the car. It uses a fuel cell instead of a combustion engine, and fuel cells are more efficient. A combustion engine converts around 20 – 40% of the energy in petrol or diesel into useful energy for the car. A fuel cell converts about 40 – 60% of the energy in hydrogen, which is better. It additionally needs an electric motor, which is about 95% efficient, but you can see that overall, a hydrogen-powered vehicle beats a petrol/diesel one in terms of using delivered energy efficiency. Unfortunately, it loses to an electric vehicle, which skips the fuel cell entirely and just uses the 95% efficient electric motor.
Let’s peel back another layer of detail. The hydrogen supplied to you at the pump can either be made with or without fossil fuels. The latter is known as green hydrogen, and it uses (green) electricity and water. This fairly expensive process is called electrolysis and it has an efficiency of about 70% [ref, ref]. That is, 100 useful units of input energy from electricity gets you about 70 useful units of output energy from hydrogen. As a side note, you’ll often hear that green hydrogen is made from water. The hydrogen itself does come from water, but the energy it supplies comes from electricity. Hydrogen is an energy carrier like electricity, not an energy source like the Sun, and this is often misunderstood. For many purposes, you can just use the electricity itself, which avoids wasting 30% of it to make hydrogen. Electric vehicles are a good example, bypassing the need for hydrogen entirely. So, not only do they skip the energy losses in fuel cells, they also skip the losses in hydrogen manufacture.
Hydrogen made from fossil fuels has a roughly similar efficiency to electrolysis, variable between 60 – 90% [ref, ref]. Most hydrogen is made this way. Most vehicles already run on fossil fuels too though, without the losses involved in hydrogen production. You could correctly bring in the greater efficiency of hydrogen fuel cells compared to combustion engines here. Assuming efficiencies towards at the top end for both hydrogen production and fuel cell operation, you’d still just about beat a conventional petrol/diesel vehicle, but again, not an electric one.
We also haven’t started to consider the energy costs in hydrogen storage and transport yet. This isn’t as simple for hydrogen as petrol or diesel, which are liquids. Hydrogen needs to be compressed and/or cooled, and these processes use additional energy. The production of liquid hydrogen is particularly energy intensive.
Together, the steps from production to end use give hydrogen a low energy efficiency compared to alternatives. I have summarised this in the diagram below, which shows how much useable energy remains after various hydrogen preparation and storage techniques, compared to an electric vehicle.
Looked at another way, each of these steps incurs an additional monetary cost, which must be paid for somewhere, and to some degree by the end user. I will return to this below, but it’s hard to believe that it wouldn’t present a considerable barrier for hydrogen in a market that already provides more established solutions.
Hydrogen as a fuel for heavier vehicles

Some transport sectors are a challenge for existing electric vehicle technologies. These are aviation, shipping, buses and long-haul HGVs. It is in these areas that the UK’s hydrogen strategy sees the most significant role for hydrogen in the transport sector. Let’s take aviation as an example to illustrate why they may be a challenge for hydrogen too. This partly comes down to its energy density.
You may see written that hydrogen has the highest energy content of any fuel we use today. Example here. This is misleading because we need to distinguish between energy content per unit mass and unit volume. Hydrogen excels at one but is bottom of the class for the other. It’s correct to say that hydrogen delivers the most energy of any fuel per kg. For the same mass of hydrogen, you get nearly three times more energy than the same mass of either gasoline or diesel: 1 kg of hydrogen contains the same energy as about 2.8 kg of petrol.
The problem is that hydrogen has a very low density compared to other fuels. In fact, it’s the lightest chemical element. This means you need a much larger volume of it to achieve the same mass as any other fuel. To match the energy in one 1 litre of petrol, you need between 3 – 14 litres of hydrogen. I’ve included a range of values here because hydrogen can either be stored as a liquid, or a gas under pressure, and this will affect the amount of energy packed into a given volume.
Hydrogen’s energy density per unit volume – that is, the amount of energy stored per unit of volume – is considerably lower than other fuels under the same conditions. There’s simply no physical way to derive the same amount of energy from a litre of hydrogen and a litre of petrol or diesel: hydrogen will always give you less. Even when compared against another gas, natural gas (methane), hydrogen gives less energy at any given pressure and volume [ref].
In many real-world contexts, it’s the volume that matters. That’s because hydrogen needs to be stored in fuel tanks, both inside vehicles and in refuelling stations. Here, hydrogen’s ability to deliver more energy per kilo is overshadowed by the amount that will fit into any given space.
You can see this in some real-world examples. Hydrogen powered vehicles, such as the Toyota Mirai and Hyundai Nexo, have multiple fuel tanks with combined volumes several times that of their gasoline or diesel counterparts. It’s typically 45 – 65 L for a petrol/diesel car compared to 122 L for the Mirai and 156 L for the Nexo.
For aviation, a similar problem occurs. To cover the same distance as a conventional plane, the space occupied by hydrogen would be around four times larger than that of kerosene [ref]. Suggested solutions for this have removed up to two-thirds of passenger seating to accommodate the tanks, and would therefore take an equivalent revenue hit with every flight [ref, ref].
Note that this estimation is for liquid hydrogen, which appears to be favoured [ref, ref] over the gas. It takes up much less space but is more energy intensive to produce. Now that we’re back at energy efficiency, let’s look at the requirements of an airport running hydrogen-powered planes. Say that 50 planes leave an airport every day, each carrying 130 tonnes of kerosene. If these energy requirements are to be matched by liquid hydrogen (= 50 tonnes), there would be a daily requirement of 2,500 tonnes of hydrogen, which has a volume of 36,000 m3, or 18 Olympic-size swimming pools [ref].
To meet its production through electrolysis, 8 GW of power would be needed to supply the electricity [ref]. The UK’s biggest power station, Drax, has an output of 4 GW [ref]. So you’d need two of them just to supply those 50 planes. In the real-world, about 1,300 flights leave Heathrow every day [ref].
Of course, we could instead produce hydrogen through steam reforming, and that starts to look quite likely, doesn’t it. Recall that our target is for at least half to be from electrolysis [ref], but let’s also consider steam reforming for completeness. We’d need carbon capture and storage (CCS) technologies for this to be sustainable, a point which is not missed by the Hydrogen Strategy. Assuming that these become operational, they’ll detract still further from hydrogen’s energy efficiency pipeline.
This brings us back to the monetary cost of all this. There’s already a well-established market for hydrogen because it’s a common chemical feedstock for industrial processes like plastic production. Pressurised gaseous hydrogen currently retails for about $15/kg in the US, and $8 – 10/kg in parts of Europe where hydrogen production is optimised [ref]. Liquid hydrogen is more expensive, as you might expect. Let’s assume that carbon-neutral hydrogen, produced from a mixture of expensive electrolysis and steam reforming with CCS, can be produced at the same price. Even at the lower end of $8/kg, this is still about four times more than the cost of kerosene, and that’s after accounting for the higher energy density per kg.
Whilst hydrogen shows potential in some sectors, not least as a possible replacement for coal in the production of steel, it looks like a problematic solution for low-carbon transport. Whilst I wouldn’t write it off entirely, it’s currently difficult to see how it will overcome some significant hurdles to become competitive.